Taking Norditropin®
FlexPro®: Meet the pen
Every journey needs a sidekick. See how FlexPro® might support you on your growth journey.

What are a few benefits of the FlexPro® pen?
Prefilled and preloadeda

William, born with GHD
Flexible storage

Ayrton, born with GHD
Easy to learn to useb
Please see Instructions for Use for complete instructions
aNeedles are sold separately and may require a prescription in some states.
bIn a study, 94 people used the Norditropin® FlexPro® 30 mg pen to inject into a foam cushion, and rated it a 6.7 out of 7 (on a scale of 1 to 7, where 1 means “strongly disagree” and 7 means “strongly agree”), as “easy to learn to use.”
FlexPro® uses one of the thinnest needles we makea
Another great thing about FlexPro®? The needles are designed to minimize pain.

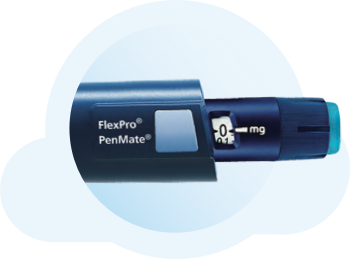
PenMate® keeps needles out of sight
PenMate® is a reusable cover for the FlexPro® 5 mg, 10 mg, and 15 mg pens.c Once you attach it to the end of your pen, it completely covers the needle so you don’t see it go into your skin during injection. PenMate® also has a large gripping surface so you can hold it firmly in your hands.
To order your PenMate®, contact your NovoCare® Case Manager or call 1-888-668-6444.
cPenMate® cannot be used with FlexPro® 30 mg pens.
Refrigeration optional after first use
New FlexPro® pens should be stored in the refrigerator. After you’ve used it once, you have options:
- Store at room temperature (up to 77 °F) for up to 3 weeksd
- Store in the fridge (36 °F to 46 °F) for up to 4 weeksd
dAll unused Norditropin® products must be refrigerated (36 °F to 46 °F) prior to first use. Do not freeze and avoid direct light. After first injection, Norditropin® pens can either be stored outside of the refrigerator (up to 77 °F) for use within 3 weeks, or in the refrigerator (between 36 °F and 46 °F) for use within 4 weeks.
Device attribute checklist
Use our tool to learn about selected attributes of the Norditropin® pen and a range of other daily and weekly growth hormone therapy devices.
This chart is not intended to be a comparison of efficacy or safety of any of these products.
This is neither a comprehensive description of each product nor a complete list of available therapies. Only select attributes of select devices are shown.
Please refer to each product's full Prescribing Information including instructions for use.
Please refer to the complete Prescribing Information for Norditropin® before use.
Please refer to the complete Prescribing Information for Sogroya® before use.
Let’s grow. Let’s go!
Free decorations? How charming!
Charms and stickers are a great way to add fun and familiarity to your FlexPro® pen. Choose from a gallery of free options!

Making growth a habit
Growth hormone therapy can become a part of your routine. Check out our tips for making it a positive and consistent one.
Important Safety Information:
Do not use Norditropin® if:
- you have a critical illness caused by certain types of heart or stomach surgery, trauma or breathing (respiratory) problems
- you are a child with Prader-Willi syndrome who is severely obese or has breathing problems including sleep apnea (briefly stop breathing during sleep)
- you have cancer or other tumors
- you are allergic to somatropin or any of the ingredients in Norditropin®
- your healthcare provider tells you that you have certain types of eye problems caused by diabetes (diabetic retinopathy)
- you are a child with closed bone growth plates (epiphyses)
Before taking Norditropin®, tell your healthcare provider about all of your medical conditions, including if you:
- have had heart or stomach surgery, trauma or serious breathing (respiratory problems)
- have had a history of problems breathing while you sleep (sleep apnea)
- have or have had cancer or any tumor
- have diabetes
- are pregnant or breastfeeding, or plan to become pregnant or breastfeed
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Norditropin® may affect how other medicines work, and other medicines may affect how Norditropin® works.
How should I use Norditropin®?
- Use Norditropin® exactly as your health care provider tells you to.
- Do not share your Norditropin® pens and needles with another person even if the needle has been changed. You may give another person an infection or get an infection from them.
What are the possible side effects of Norditropin®?
Norditropin® may cause serious side effects, including:
- high risk of death in people who have critical illnesses because of heart or stomach surgery, trauma or serious breathing (respiratory) problems
- high risk of sudden death in children with Prader-Willi syndrome who are severely obese or have breathing problems including sleep apnea
- increased risk of growth of cancer or a tumor that is already present and increased risk of the return of cancer or a tumor in people who were treated with radiation to the brain or head as children and who developed low growth hormone problems. Contact the healthcare provider if you or your child start to have headaches, or have changes in behavior, changes in vision, or changes in moles, birthmarks, or the color of your skin
- new or worsening high blood sugar (hyperglycemia) or diabetes
- increase in pressure in the skull (intracranial hypertension). If you or your child has headaches, eye problems, nausea or vomiting, contact the healthcare provider
- serious allergic reactions. Get medical help right away if you or your child has the following symptoms: swelling of your face, lips, mouth, or tongue, trouble breathing, wheezing, severe itching, skin rashes, redness or swelling, dizziness or fainting, fast heartbeat or pounding in your chest, or sweating
- your body holding too much fluid (fluid retention) such as swelling in the hands and feet, pain in your joints or muscles or nerve problems that cause pain, burning, or tingling in the hands, arms, legs and feet. Tell your healthcare provider if you have any of these signs or symptoms of fluid retention
- decrease in a hormone called cortisol. Tell your or your child’s healthcare provider if you or your child has darkening of the skin, severe fatigue, dizziness, weakness, or weight loss
- decrease in thyroid hormone levels
- hip and knee pain or a limp in children (slipped capital femoral epiphysis). This may lead to a serious condition where bone tissue dies due to a lack of blood supply (osteonecrosis). Get medical help right away if your child develops a limp or has hip or knee pain
- worsening of curvature of the spine (scoliosis)
- severe and constant abdominal pain can be a sign of pancreatitis. Tell your or your child’s healthcare provider if you or your child has any new abdominal pain
- loss of fat and tissue weakness in the area of skin you inject
- increase in phosphorus, alkaline phosphatase, and parathyroid hormone levels in your blood
The most common side effects of Norditropin® include:
- injection site reactions and rashes, and headaches
Please click here for Norditropin® Prescribing Information.
Norditropin® is a prescription medication. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
What is Norditropin®?
Norditropin® (somatropin) injection 5 mg, 10 mg, 15 mg, or 30 mg is a prescription medicine that contains human growth hormone, the same growth hormone made by the human body.
Norditropin® is given under the skin (subcutaneous) and is used to treat:
- children who are not growing because of low or no growth hormone
- children who are short (in stature) and who have Noonan syndrome, Turner syndrome, or were born small (small for gestational age-SGA) and have not caught-up in growth by age 2 to 4 years
- children who have Idiopathic Short Stature (ISS)
- children who are not growing who have Prader-Willi syndrome (PWS)
- adults who do not make enough growth hormone
Important Safety Information
Do not use Sogroya® if:
- you have a critical illness caused by certain types of heart or stomach surgery, trauma or breathing problems
- you have cancer or other tumors
- you are allergic to somapacitan-beco or any of the ingredients in Sogroya®
- your healthcare provider tells you that you have certain types of eye problems caused by diabetes
- you are a child with closed bone growth plates
- you are a child with Prader-Willi syndrome who is severely obese or has breathing problems including sleep apnea (briefly stopping breathing during sleep)
Before taking Sogroya®, tell your healthcare provider about all of your medical conditions, including if you:
- have had heart or stomach surgery, trauma, or serious breathing problems
- are taking replacement therapy with glucocorticoids
- have had cancer or any tumor
- have thyroid gland problems
- have diabetes
- have liver problems
- have adrenal gland problems
- are a child with a history of worsening of curvature of the spine (scoliosis)
- are pregnant or plan to become pregnant. It is not known if Sogroya® will harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant
- are breastfeeding or plan to breastfeed. It is not known if Sogroya® passes into your breast milk. You and your healthcare provider should decide if you will take Sogroya® while you breastfeed
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Sogroya® may affect the way some medicines work, and some medicines may affect how Sogroya® works.
How should I use Sogroya®?
- Use Sogroya® exactly as your healthcare provider tells you to
- Use Sogroya® 1 time each week
- If you miss a dose of Sogroya®, the missed dose can be taken within 3 days (72 hours) after the scheduled dosing day. One-time weekly dosing for the next dose can be started again on the regularly scheduled dosing day
- If more than 3 days (72 hours) have passed, skip the missed dose, and take your next dose on the regularly scheduled dosing day
- Sogroya® pens are for use by 1 person only
- Do not share your Sogroya® pens and needles with another person, even if the needle has been changed. You may give another person an infection or get an infection from them
What are the possible side effects of Sogroya®?
Sogroya® may cause serious side effects, including:
- high risk of death in people who have critical illnesses because of heart or stomach surgery, trauma, or serious breathing problems
- increased risk of growth of cancer or a tumor that is already present and increased risk of the return of cancer or a tumor in people who were treated with radiation to the brain or head as children and who developed low growth hormone problems. You or your child’s healthcare provider will need to monitor you or your child for a return of cancer or a tumor. Contact the healthcare provider if you or your child start to have sudden changes in behavior, headaches, vision problems, or changes in moles, birthmarks, or the color of your or your child’s skin
- new or worsening high blood sugar or diabetes. You or your child’s blood sugar may need to be monitored during treatment with Sogroya®
- increase in pressure in the skull. If you or your child have headaches, eye problems, nausea or vomiting, contact the healthcare provider
- serious allergic reactions. Get medical help right away if you or your child have the following symptoms: swelling of your face, lips, mouth, or tongue, trouble breathing, wheezing, severe itching, skin rashes, redness, or swelling, dizziness or fainting, fast heartbeat or pounding in your chest, sweating
- your or your child’s body holding too much fluid (fluid retention) such as swelling in the hands and feet, pain in your or your child’s joints or muscles or nerve problems that cause pain, burning or tingling in the hands, arms, legs, and feet. Tell your or your child’s healthcare provider if you or your child have any of these signs or symptoms of fluid retention
- decrease in a hormone called cortisol. The healthcare provider will do blood tests to check your or your child’s cortisol levels. Tell your or your child’s healthcare provider if you or your child have darkening of the skin, severe fatigue, dizziness, weakness, or weight loss
- decrease in thyroid hormone levels. Decreased thyroid hormone levels may affect how well Sogroya® works. The healthcare provider will do blood tests to check you or your child’s thyroid hormone levels
- severe and constant abdominal pain. This could be a sign of pancreatitis. Tell your or your child’s healthcare provider if you or your child has any new abdominal pain
- loss of fat and tissue weakness in the area of skin you or your child inject. Talk to your or your child’s healthcare provider about rotating the areas where you or your child inject Sogroya®
- worsening of curvature of the spine in children (scoliosis)
- hip and knee pain or a limp in children (slipped capital femoral epiphysis). This may lead to a serious condition where bone tissue dies due to lack of blood supply (osteonecrosis). Get medical help right away if your child develops a limp or has hip or knee pain
- high risk of sudden death in children with Prader-Willi syndrome who are severely obese or have breathing problems, including sleep apnea
- increase in phosphorus, alkaline phosphatase, and parathyroid hormone levels in your blood. You or your child’s healthcare provider will do blood tests to check this
The most common side effects of Sogroya® in children include: common cold, headache, fever, pain in extremity, and reaction to injection
The most common side effects of Sogroya® in adults include: back pain, joint pain, indigestion, sleep problems, dizziness, swelling of the tonsils (tonsillitis), vomiting, high blood pressure, increase in the level of an enzyme in your blood called creatine phosphokinase, weight gain, and low red blood cells (anemia)
Please click here for Sogroya® Prescribing Information.
Sogroya® is a prescription medication. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
What is Sogroya®?
- Sogroya® (somapacitan-beco) injection 5 mg, 10 mg or 15 mg is a prescription medicine that contains human growth hormone, the same growth hormone made by the body.
- It is given by injection under the skin (subcutaneous) and is used to treat adults and children 2.5 years and older who do not make enough growth hormone.
What is Norditropin®?
Norditropin® (somatropin) injection 5 mg, 10 mg, 15 mg, or 30 mg is a prescription medicine that contains human growth hormone, the same growth hormone made by the human body.
Norditropin® is given under the skin (subcutaneous) and is used to treat:
- children who are not growing because of low or no growth hormone
- children who are short (in stature) and who have Noonan syndrome, Turner syndrome, or were born small (small for gestational age-SGA) and have not caught-up in growth by age 2 to 4 years
- children who have Idiopathic Short Stature (ISS)
- children who are not growing who have Prader-Willi syndrome (PWS)
- adults who do not make enough growth hormone
Important Safety Information:
Do not use Norditropin® if:
- you have a critical illness caused by certain types of heart or stomach surgery, trauma or breathing (respiratory) problems
- you are a child with Prader-Willi syndrome who is severely obese or has breathing problems including sleep apnea (briefly stop breathing during sleep)
- you have cancer or other tumors
- you are allergic to somatropin or any of the ingredients in Norditropin®
- your healthcare provider tells you that you have certain types of eye problems caused by diabetes (diabetic retinopathy)
- you are a child with closed bone growth plates (epiphyses)
Before taking Norditropin®, tell your healthcare provider about all of your medical conditions, including if you:
- have had heart or stomach surgery, trauma or serious breathing (respiratory problems)
- have had a history of problems breathing while you sleep (sleep apnea)
- have or have had cancer or any tumor
- have diabetes
- are pregnant or breastfeeding, or plan to become pregnant or breastfeed
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Norditropin® may affect how other medicines work, and other medicines may affect how Norditropin® works.
How should I use Norditropin®?
- Use Norditropin® exactly as your health care provider tells you to.
- Do not share your Norditropin® pens and needles with another person even if the needle has been changed. You may give another person an infection or get an infection from them.
What is Sogroya®?
- Sogroya® (somapacitan-beco) injection 5 mg, 10 mg or 15 mg is a prescription medicine that contains human growth hormone, the same growth hormone made by the body.
- It is given by injection under the skin (subcutaneous) and is used to treat adults and children 2.5 years and older who do not make enough growth hormone.
Important Safety Information
Do not use Sogroya® if:
- you have a critical illness caused by certain types of heart or stomach surgery, trauma or breathing problems
- you have cancer or other tumors
- you are allergic to somapacitan-beco or any of the ingredients in Sogroya®
- your healthcare provider tells you that you have certain types of eye problems caused by diabetes
- you are a child with closed bone growth plates
- you are a child with Prader-Willi syndrome who is severely obese or has breathing problems including sleep apnea (briefly stopping breathing during sleep)
Before taking Sogroya®, tell your healthcare provider about all of your medical conditions, including if you:
- have had heart or stomach surgery, trauma, or serious breathing problems
- are taking replacement therapy with glucocorticoids
- have had cancer or any tumor
- have thyroid gland problems
- have diabetes
- have liver problems
- have adrenal gland problems
- are a child with a history of worsening of curvature of the spine (scoliosis)
- are pregnant or plan to become pregnant. It is not known if Sogroya® will harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant
- are breastfeeding or plan to breastfeed. It is not known if Sogroya® passes into your breast milk. You and your healthcare provider should decide if you will take Sogroya® while you breastfeed
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Sogroya® may affect the way some medicines work, and some medicines may affect how Sogroya® works.
How should I use Sogroya®?
- Use Sogroya® exactly as your healthcare provider tells you to
- Use Sogroya® 1 time each week
- If you miss a dose of Sogroya®, the missed dose can be taken within 3 days (72 hours) after the scheduled dosing day. One-time weekly dosing for the next dose can be started again on the regularly scheduled dosing day
- If more than 3 days (72 hours) have passed, skip the missed dose, and take your next dose on the regularly scheduled dosing day
- Sogroya® pens are for use by 1 person only
- Do not share your Sogroya® pens and needles with another person, even if the needle has been changed. You may give another person an infection or get an infection from them
What are the possible side effects of Sogroya®?
Sogroya® may cause serious side effects, including:
- high risk of death in people who have critical illnesses because of heart or stomach surgery, trauma, or serious breathing problems
- increased risk of growth of cancer or a tumor that is already present and increased risk of the return of cancer or a tumor in people who were treated with radiation to the brain or head as children and who developed low growth hormone problems. You or your child’s healthcare provider will need to monitor you or your child for a return of cancer or a tumor. Contact the healthcare provider if you or your child start to have sudden changes in behavior, headaches, vision problems, or changes in moles, birthmarks, or the color of your or your child’s skin
- new or worsening high blood sugar or diabetes. You or your child’s blood sugar may need to be monitored during treatment with Sogroya®
- increase in pressure in the skull. If you or your child have headaches, eye problems, nausea or vomiting, contact the healthcare provider
- serious allergic reactions. Get medical help right away if you or your child have the following symptoms: swelling of your face, lips, mouth, or tongue, trouble breathing, wheezing, severe itching, skin rashes, redness, or swelling, dizziness or fainting, fast heartbeat or pounding in your chest, sweating
- your or your child’s body holding too much fluid (fluid retention) such as swelling in the hands and feet, pain in your or your child’s joints or muscles or nerve problems that cause pain, burning or tingling in the hands, arms, legs, and feet. Tell your or your child’s healthcare provider if you or your child have any of these signs or symptoms of fluid retention
- decrease in a hormone called cortisol. The healthcare provider will do blood tests to check your or your child’s cortisol levels. Tell your or your child’s healthcare provider if you or your child have darkening of the skin, severe fatigue, dizziness, weakness, or weight loss
- decrease in thyroid hormone levels. Decreased thyroid hormone levels may affect how well Sogroya® works. The healthcare provider will do blood tests to check you or your child’s thyroid hormone levels
- severe and constant abdominal pain. This could be a sign of pancreatitis. Tell your or your child’s healthcare provider if you or your child has any new abdominal pain
- loss of fat and tissue weakness in the area of skin you or your child inject. Talk to your or your child’s healthcare provider about rotating the areas where you or your child inject Sogroya®
- worsening of curvature of the spine in children (scoliosis)
- hip and knee pain or a limp in children (slipped capital femoral epiphysis). This may lead to a serious condition where bone tissue dies due to lack of blood supply (osteonecrosis). Get medical help right away if your child develops a limp or has hip or knee pain
- high risk of sudden death in children with Prader-Willi syndrome who are severely obese or have breathing problems, including sleep apnea
- increase in phosphorus, alkaline phosphatase, and parathyroid hormone levels in your blood. You or your child’s healthcare provider will do blood tests to check this
The most common side effects of Sogroya® in children include: common cold, headache, fever, pain in extremity, and reaction to injection
The most common side effects of Sogroya® in adults include: back pain, joint pain, indigestion, sleep problems, dizziness, swelling of the tonsils (tonsillitis), vomiting, high blood pressure, increase in the level of an enzyme in your blood called creatine phosphokinase, weight gain, and low red blood cells (anemia)
Please click here for Sogroya® Prescribing Information.
Sogroya® is a prescription medication. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
Important Safety Information:
Do not use Norditropin® if:
- you have a critical illness caused by certain types of heart or stomach surgery, trauma or breathing (respiratory) problems
- you are a child with Prader-Willi syndrome who is severely obese or has breathing problems including sleep apnea (briefly stop breathing during sleep)
- you have cancer or other tumors
- you are allergic to somatropin or any of the ingredients in Norditropin®
- your healthcare provider tells you that you have certain types of eye problems caused by diabetes (diabetic retinopathy)
- you are a child with closed bone growth plates (epiphyses)
Before taking Norditropin®, tell your healthcare provider about all of your medical conditions, including if you:
- have had heart or stomach surgery, trauma or serious breathing (respiratory problems)
- have had a history of problems breathing while you sleep (sleep apnea)
- have or have had cancer or any tumor
- have diabetes
- are pregnant or breastfeeding, or plan to become pregnant or breastfeed
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Norditropin® may affect how other medicines work, and other medicines may affect how Norditropin® works.
How should I use Norditropin®?
- Use Norditropin® exactly as your health care provider tells you to.
- Do not share your Norditropin® pens and needles with another person even if the needle has been changed. You may give another person an infection or get an infection from them.
What are the possible side effects of Norditropin®?
Norditropin® may cause serious side effects, including:
- high risk of death in people who have critical illnesses because of heart or stomach surgery, trauma or serious breathing (respiratory) problems
- high risk of sudden death in children with Prader-Willi syndrome who are severely obese or have breathing problems including sleep apnea
- increased risk of growth of cancer or a tumor that is already present and increased risk of the return of cancer or a tumor in people who were treated with radiation to the brain or head as children and who developed low growth hormone problems. Contact the healthcare provider if you or your child start to have headaches, or have changes in behavior, changes in vision, or changes in moles, birthmarks, or the color of your skin
- new or worsening high blood sugar (hyperglycemia) or diabetes
- increase in pressure in the skull (intracranial hypertension). If you or your child has headaches, eye problems, nausea or vomiting, contact the healthcare provider
- serious allergic reactions. Get medical help right away if you or your child has the following symptoms: swelling of your face, lips, mouth, or tongue, trouble breathing, wheezing, severe itching, skin rashes, redness or swelling, dizziness or fainting, fast heartbeat or pounding in your chest, or sweating
- your body holding too much fluid (fluid retention) such as swelling in the hands and feet, pain in your joints or muscles or nerve problems that cause pain, burning, or tingling in the hands, arms, legs and feet. Tell your healthcare provider if you have any of these signs or symptoms of fluid retention
- decrease in a hormone called cortisol. Tell your or your child’s healthcare provider if you or your child has darkening of the skin, severe fatigue, dizziness, weakness, or weight loss
- decrease in thyroid hormone levels
- hip and knee pain or a limp in children (slipped capital femoral epiphysis). This may lead to a serious condition where bone tissue dies due to a lack of blood supply (osteonecrosis). Get medical help right away if your child develops a limp or has hip or knee pain
- worsening of curvature of the spine (scoliosis)
- severe and constant abdominal pain can be a sign of pancreatitis. Tell your or your child’s healthcare provider if you or your child has any new abdominal pain
- loss of fat and tissue weakness in the area of skin you inject
- increase in phosphorus, alkaline phosphatase, and parathyroid hormone levels in your blood
The most common side effects of Norditropin® include:
- injection site reactions and rashes, and headaches
Please click here for Norditropin® Prescribing Information.
Norditropin® is a prescription medication. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
What is Norditropin®?
Norditropin® (somatropin) injection 5 mg, 10 mg, 15 mg, or 30 mg is a prescription medicine that contains human growth hormone, the same growth hormone made by the human body.
Norditropin® is given under the skin (subcutaneous) and is used to treat:
- children who are not growing because of low or no growth hormone
- children who are short (in stature) and who have Noonan syndrome, Turner syndrome, or were born small (small for gestational age-SGA) and have not caught-up in growth by age 2 to 4 years
- children who have Idiopathic Short Stature (ISS)
- children who are not growing who have Prader-Willi syndrome (PWS)
- adults who do not make enough growth hormone
Important Safety Information
Do not use Sogroya® if:
- you have a critical illness caused by certain types of heart or stomach surgery, trauma or breathing problems
- you have cancer or other tumors
- you are allergic to somapacitan-beco or any of the ingredients in Sogroya®
- your healthcare provider tells you that you have certain types of eye problems caused by diabetes
- you are a child with closed bone growth plates
- you are a child with Prader-Willi syndrome who is severely obese or has breathing problems including sleep apnea (briefly stopping breathing during sleep)
Before taking Sogroya®, tell your healthcare provider about all of your medical conditions, including if you:
- have had heart or stomach surgery, trauma, or serious breathing problems
- are taking replacement therapy with glucocorticoids
- have had cancer or any tumor
- have thyroid gland problems
- have diabetes
- have liver problems
- have adrenal gland problems
- are a child with a history of worsening of curvature of the spine (scoliosis)
- are pregnant or plan to become pregnant. It is not known if Sogroya® will harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant
- are breastfeeding or plan to breastfeed. It is not known if Sogroya® passes into your breast milk. You and your healthcare provider should decide if you will take Sogroya® while you breastfeed
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Sogroya® may affect the way some medicines work, and some medicines may affect how Sogroya® works.
How should I use Sogroya®?
- Use Sogroya® exactly as your healthcare provider tells you to
- Use Sogroya® 1 time each week
- If you miss a dose of Sogroya®, the missed dose can be taken within 3 days (72 hours) after the scheduled dosing day. One-time weekly dosing for the next dose can be started again on the regularly scheduled dosing day
- If more than 3 days (72 hours) have passed, skip the missed dose, and take your next dose on the regularly scheduled dosing day
- Sogroya® pens are for use by 1 person only
- Do not share your Sogroya® pens and needles with another person, even if the needle has been changed. You may give another person an infection or get an infection from them
What are the possible side effects of Sogroya®?
Sogroya® may cause serious side effects, including:
- high risk of death in people who have critical illnesses because of heart or stomach surgery, trauma, or serious breathing problems
- increased risk of growth of cancer or a tumor that is already present and increased risk of the return of cancer or a tumor in people who were treated with radiation to the brain or head as children and who developed low growth hormone problems. You or your child’s healthcare provider will need to monitor you or your child for a return of cancer or a tumor. Contact the healthcare provider if you or your child start to have sudden changes in behavior, headaches, vision problems, or changes in moles, birthmarks, or the color of your or your child’s skin
- new or worsening high blood sugar or diabetes. You or your child’s blood sugar may need to be monitored during treatment with Sogroya®
- increase in pressure in the skull. If you or your child have headaches, eye problems, nausea or vomiting, contact the healthcare provider
- serious allergic reactions. Get medical help right away if you or your child have the following symptoms: swelling of your face, lips, mouth, or tongue, trouble breathing, wheezing, severe itching, skin rashes, redness, or swelling, dizziness or fainting, fast heartbeat or pounding in your chest, sweating
- your or your child’s body holding too much fluid (fluid retention) such as swelling in the hands and feet, pain in your or your child’s joints or muscles or nerve problems that cause pain, burning or tingling in the hands, arms, legs, and feet. Tell your or your child’s healthcare provider if you or your child have any of these signs or symptoms of fluid retention
- decrease in a hormone called cortisol. The healthcare provider will do blood tests to check your or your child’s cortisol levels. Tell your or your child’s healthcare provider if you or your child have darkening of the skin, severe fatigue, dizziness, weakness, or weight loss
- decrease in thyroid hormone levels. Decreased thyroid hormone levels may affect how well Sogroya® works. The healthcare provider will do blood tests to check you or your child’s thyroid hormone levels
- severe and constant abdominal pain. This could be a sign of pancreatitis. Tell your or your child’s healthcare provider if you or your child has any new abdominal pain
- loss of fat and tissue weakness in the area of skin you or your child inject. Talk to your or your child’s healthcare provider about rotating the areas where you or your child inject Sogroya®
- worsening of curvature of the spine in children (scoliosis)
- hip and knee pain or a limp in children (slipped capital femoral epiphysis). This may lead to a serious condition where bone tissue dies due to lack of blood supply (osteonecrosis). Get medical help right away if your child develops a limp or has hip or knee pain
- high risk of sudden death in children with Prader-Willi syndrome who are severely obese or have breathing problems, including sleep apnea
- increase in phosphorus, alkaline phosphatase, and parathyroid hormone levels in your blood. You or your child’s healthcare provider will do blood tests to check this
The most common side effects of Sogroya® in children include: common cold, headache, fever, pain in extremity, and reaction to injection
The most common side effects of Sogroya® in adults include: back pain, joint pain, indigestion, sleep problems, dizziness, swelling of the tonsils (tonsillitis), vomiting, high blood pressure, increase in the level of an enzyme in your blood called creatine phosphokinase, weight gain, and low red blood cells (anemia)
Please click here for Sogroya® Prescribing Information.
Sogroya® is a prescription medication. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
What is Sogroya®?
- Sogroya® (somapacitan-beco) injection 5 mg, 10 mg or 15 mg is a prescription medicine that contains human growth hormone, the same growth hormone made by the body.
- It is given by injection under the skin (subcutaneous) and is used to treat adults and children 2.5 years and older who do not make enough growth hormone.
